Disaster Triage, Prioritizing, and Delegation
4 Topics | 2 Quizzes
Neurological System
10 Topics | 1 Quiz
MS, MG, and Guillain Barré
4 Topics | 1 Quiz
The Cardiovascular System
12 Topics | 1 Quiz
Pulmonary System
10 Topics | 1 Quiz
Cushing Versus Addison Disease
3 Topics | 1 Quiz
Thyroid Disorders
2 Topics | 1 Quiz
Parathyroid Disorders
3 Topics | 1 Quiz
DI and SIADH
3 Topics | 1 Quiz
Diabetes
10 Topics | 1 Quiz
Burns
5 Topics | 1 Quiz
Anemias, Aplastic Anemia, Polycythemia Vera, Thrombocytopenia and DIC
9 Topics | 1 Quiz
Cancer, Chemotherapy, Radiation Therapy, and Oncological Emergencies
6 Topics | 1 Quiz
Leukemias, Hodgkin’s Disease, and Multiple Myeloma
4 Topics | 1 Quiz
The GI system
16 Topics | 1 Quiz
Renal and Genitourinary Problems
6 Topics | 1 Quiz
Infection and Isolation Precautions
3 Topics | 1 Quiz
NCLEX Pharmacology
3 Topics | 1 Quiz
TPN, IV Solutions, & Blood Products
6 Topics | 1 Quiz
Lab Values
9 Topics
ABGs
Let's start by learning normal values
pH = 7.35 – 7.45
pCO²= 35-45 mm Hg
PaO2=80 -100 mm Hg
HCO3 = 22 – 26 mEq/L
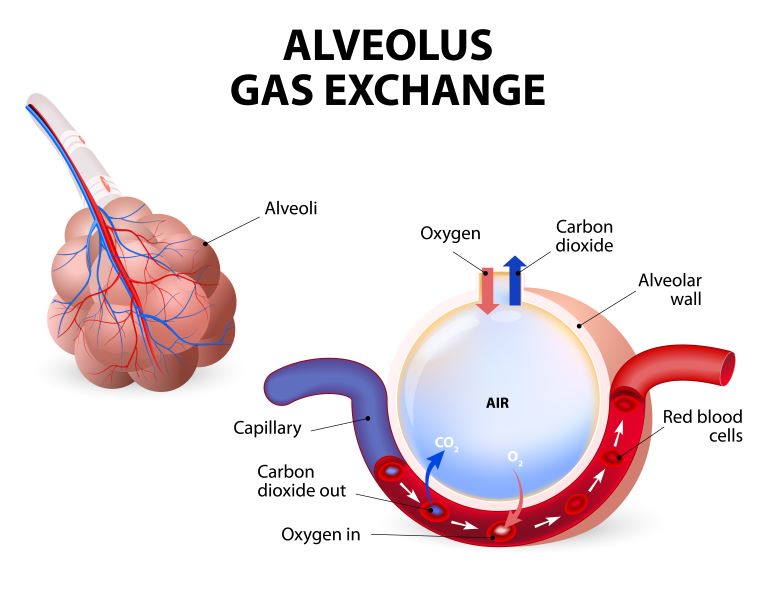
- As the person inhales, the person inhales oxygen and exhales CO2. The faster a person inhales and exhales, the more CO2 is exhaled and the lower the CO2 level in the blood.
- Fast, deep breathing lowers pCO2. CO2 is blown off.
- Slow, Shallow breathing increases pCO2. CO2 is retained.
- Consider CO2 as an ACID. Both have a “C”. High CO2 = acidosis. More specifically, respiratory acidosis
pH
< 7.35 = Acidosis
>7.45 = Alkalosis
pCO²
< 35 = Alkalosis
> 45 = Acidosis
HCO3
< 22 = Acidosis
> 26 = Alkalosis
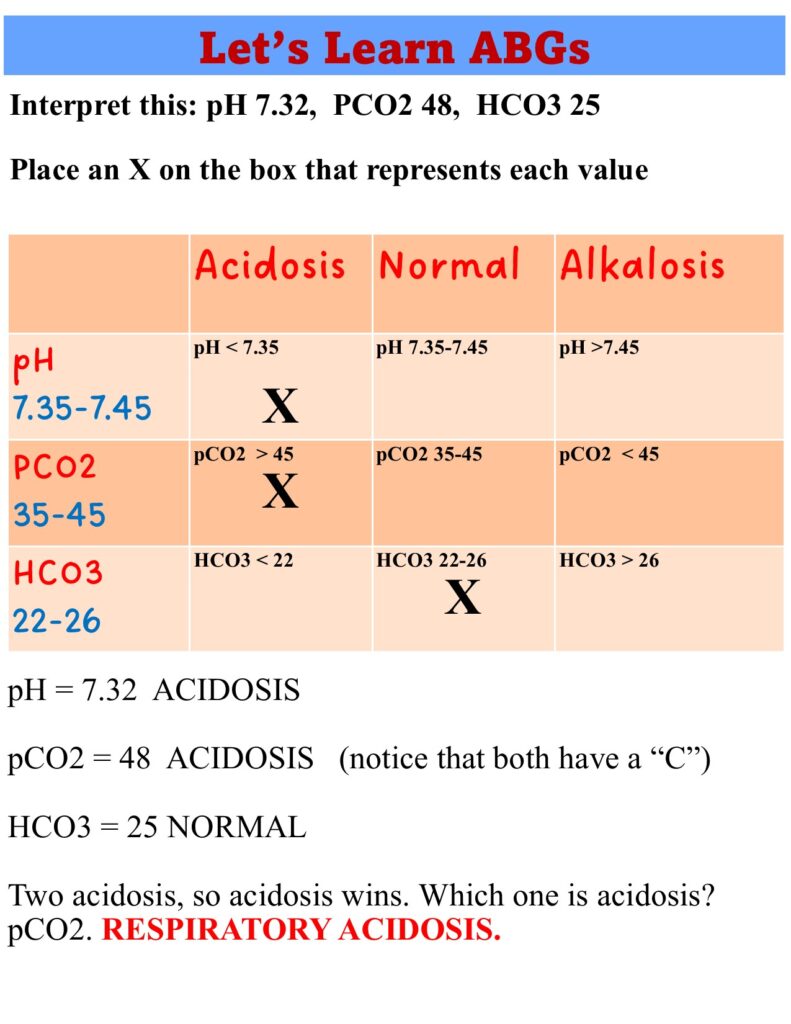
Compensated, Uncompensated and Partially Compensated ABGs
- pH =7.4
- PCO²=50
- HCO3 = 32
- Compensated respiratory acidosis: The pH is normal. For example, the client is in chronic respiratory acidosis due to COPD, a chronic respiratory disorder, and over time the kidneys have compensated by increasing the excretion of H+ and the retention of HCO3. The HCO3 levels go up.
- Compensated respiratory acidosis: Normal pH, elevated PCO2, and elevated HCO3.
- pH = 7.33
- PCO2 = 50
- HCO3 = 28
- Partially compensated respiratory acidosis. Notice that this ABG is respiratory acidosis, but the HCO3 is elevated. The kidneys are on their way to normalize pH to “compensate” for the acidosis. Give it a few days and this will turn into a compensated respiratory acidosis. As the HCO3 increases and pH will be normal (7.35 to 7.45).
- Partially compensated respiratory acidosis: Low pH, elevated PCO2, and elevated HCO3. HCO3 is increasing to balance off the acid (CO2) and normalize the base. Not there yet, but it will be.
- pH = 7.30
- PCO2 = 50
- HCO3 = 22
- Uncompensated respiratory acidosis. pH is low and PCO2 is high. The HCO3 level is on the low normal. The kidneys have done nothing to compensate for the acidosis.
- Uncompensated respiratory acidosis: Low pH, high PCO2, and normal HCO3.
- pH = 7.35
- pCO2 = 33
- HCO3 = 17
- Compensated metabolic acidosis. pH is normal so we have a compensation. Note that pCO2 is is low (alkalosis) and HCO3 is acidotic. The pH leans towards acidotic side. The client has metabolic acidosis. The client is breathing fast to blow off pCO2 and compensate for the acidosis.
- Compensated metabolic acidosis: Normal pH, Low HCO3, and Low CO2
- pH = 7.33
- pCO2 = 38
- HCO3 = 17
- Uncompensated metabolic acidosis. pH is acidotic. Note that pCO2 is normal and HCO3 is acidotic. There is no compensation, so uncompensated metabolic acidosis. If the client begins to breathe fast, the CO2 will go down and normalize the pH to compensate.
- pH = 7.33
- pCO2 = 30
- HCO3 = 15
- Partially compensated metabolic acidosis. pH is acidotic. Note that pCO2 is alkalotic and HCO3 is acidotic. The lungs are trying to compensate by blowing off pCO2 to neutralize the metabolic acidosis.
- pH = 7.45
- pCO2 = 50
- HCO3 = 30
- Compensated metabolic alkalosis. pH is normal towards the alkalotic side. Note that pCO2 is acidotic and HCO3 is alkalotic The lungs are trying to compensate by retaining pCO2 to neutralize the metabolic alkalosis. It is all about balance.
- pH = 7.46
- pCO2 = 44
- HCO3 = 28
- Uncompensated metabolic alkalosis. pH is on the alkalotic side. Note that pCO2 is normal and HCO3 is alkalotic The lungs are NOT trying to compensate.
- pH = 7.55
- pCO2 = 50
- HCO3 = 32
- Partially compensated metabolic alkalosis. pH is on the alkalotic side. Note that pCO2 is acidotic since it is trying to neutralize the alkalotic HCO3. The lungs are trying to compensate for the metabolic alkalosis, but are not there yet.
Let's play a game. Is it acidosis or alkalosis?
Drawing an ABG
- Use a heparinized tube/syringe
- No bubbles in syringe (it is seen as oxygen and pO2 will not be accurate).
- Put on ice immediately
- Label: Client’s info and whether client was on room air or oxygen (how much?)
- Do Allen’s test prior to doing ABG to check for sufficient blood flow to hand. You do not want to damage an artery to a hand that is already compromised with poor blood flow!

Allen's test
Flash Cards! Click on the headings to uncover the content
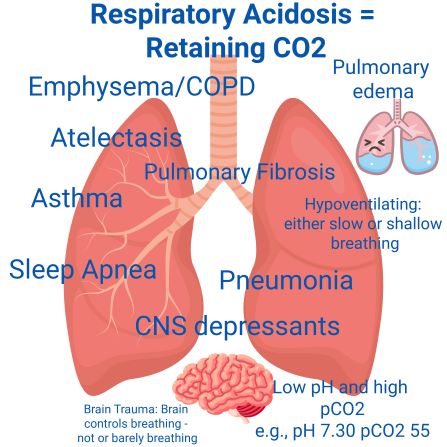
- Hypoventilating
- ⇓ Respiratory rate
- Shallow breathing
- Most, if not all, pulmonary diseases cause respiratory acidosis!
- ⇓ pH ⇑ pCO2
- You stop breathing= acidosis since retaining pCO2
- You can’t take a deep breath due to airway inflammation, secretions, chest trauma = retaning pCO2 = respiratory acidosis

- ⇑ pH and ⇓ pCO2
- Every time you breath you blow off CO2. The faster and the deeper you breath, the lower the CO2
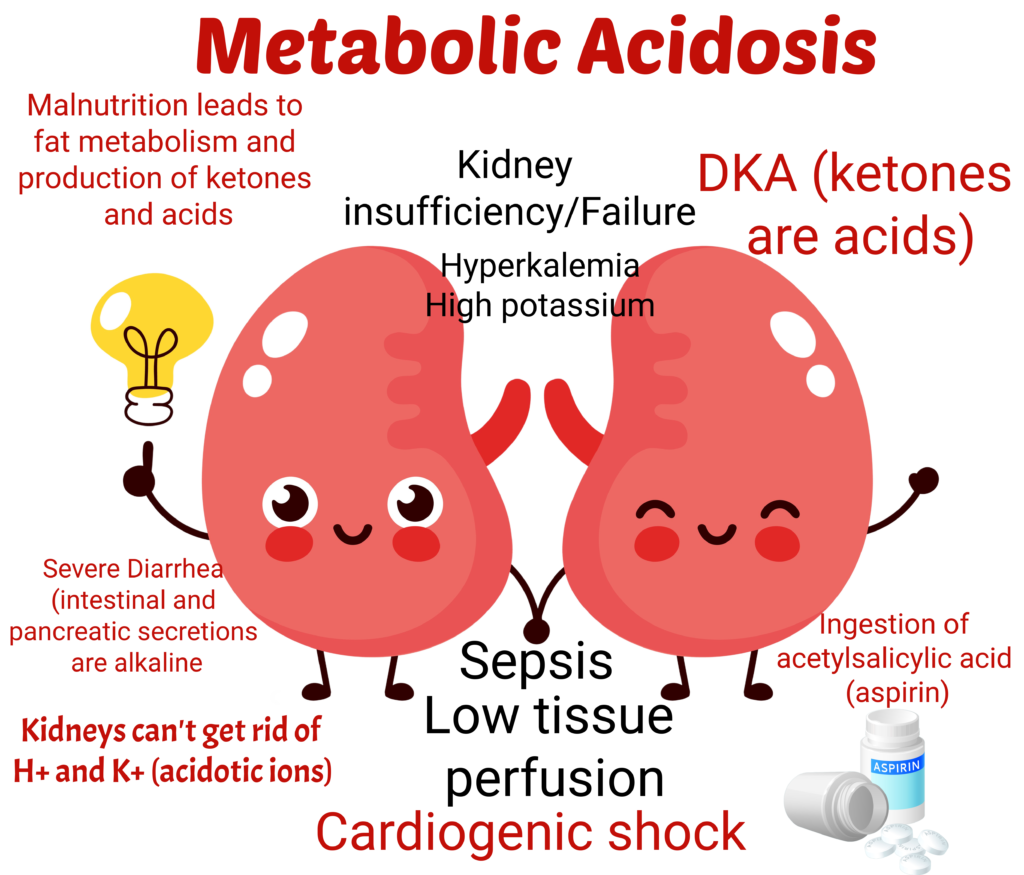
- Don’t forget that K+ ⇑ with metabolic acidosis.
- Acidosis is just as deadly as hyperkalemia. They are friends and come together
- Acidosis = low bicarbonate = Low pH

- You lose acid, you become more alkaline
- Diuretics get rid of potassium and H+ ions ⇒ loss of acid ions ⇒ Alkalosis
- You know that acidosis and alkalosis are opposites, right?
- You lose acids, you become alkalotic
- Remember that gastric secretions are acid. You lose gastric secretions (acid) now you are alkalotic.
